V6.5 Filter Integrity Tester Integtest
V6.5 Filter Integrity Tester Integtest Specification
- Frequency
- 50/60 Hz
- Max Height
- 240 mm
- Application
- Integrity testing of hydrophilic and hydrophobic membrane filters
- Test Range
- 0-6000 mL/min
- Power Supply
- AC 220V 10%, 50/60 Hz
- Measuring Range
- 0-6000 mL/min (air flow)
- Port Size
- 6 mm, 8 mm, 12 mm (customizable)
- Mounting Type
- Tabletop
- Temperature
- 5C - 40C
- Response Time
- 1 second
- Humidity
- 85% RH (non-condensing)
- Interface Type
- USB, RS232, Ethernet
- Gas Pressure
- 0.1-0.4 MPa (clean compressed air or nitrogen)
- Resolution
- 0.01 mL/min
- Specimen Size
- Various (customizable adapters)
- Accuracy
- 2% F.S.
- Equipment Type
- Automatic filter integrity tester
- Automation Grade
- Fully automatic
- Features
- Automatic data storage, printout, password protection, CFR21 compliance
- Operating Voltage
- AC 220V 10%, 50/60 Hz
- Number of Specimens
- Single filter or filter assembly
- Display Type
- 7-inch Color Touchscreen Display
- Usage
- Laboratory integrity test, pharmaceutical QC
- Machine Weight
- 9 kg
- Test Speed
- 60 seconds per test
- Control Mode
- Touchscreen Interface
- Compliance
- CFR21 Part 11, GMP, GLP
- Calibration
- Automatic and manual
- Dimensions
- 305 x 240 x 150 mm
- Printing Capability
- Built-in thermal printer
- Noise Level
- 60 dB
- Display Resolution
- 800 x 480 pixels
- Language Support
- English, Chinese (multilingual options)
- Protection Level
- IP21
- Testing Methods
- Bubble Point Test, Diffusion Test, Water Intrusion Test
- Data Storage
- Up to 100,000 test records
About V6.5 Filter Integrity Tester Integtest
Application Area:
Pharmaceutical industry
Medical device industry
Food and beverage industry
Environmental protection industry
Advanced Testing Technology
The V6.5 Filter Integrity Tester incorporates state-of-the-art automation and high-precision sensors to deliver reliable and reproducible results. With user-selectable testing methods-Bubble Point, Diffusion, and Water Intrusion-it caters to diverse filter applications. Customizable adapters make it versatile for a wide range of specimen sizes and assemblies.
User-Friendly and Secure Operation
Equipped with a 7-inch color touchscreen, the tester supports intuitive operations in English and Chinese. The device allows automatic data storage and password-protected access, ensuring compliance with strict industry regulations like CFR21 Part 11. Its thermal printer provides instant documentation of test outcomes.
Flexible and Efficient Integration
Compatible with USB, RS232, and Ethernet interfaces, the V6.5 Filter Integrity Tester integrates seamlessly into laboratory and pharmaceutical quality control systems. With rapid test speeds (60 seconds per test) and the capacity to handle up to 100,000 records, it is a powerful asset for busy QA/QC teams.
FAQ's of V6.5 Filter Integrity Tester Integtest:
Q: How does the V6.5 Filter Integrity Tester operate during filter testing?
A: The tester uses automated processes to perform Bubble Point, Diffusion, and Water Intrusion tests. The operator simply selects the desired method and test parameters via the touchscreen interface. The system then applies clean compressed air or nitrogen at precise pressures, measuring filter integrity within 60 seconds per test.Q: What types of filters can be tested with this equipment?
A: The V6.5 is designed for both hydrophilic and hydrophobic membrane filters and accommodates single filters or filter assemblies. Customizable adapters allow testing of various specimen sizes, making it suitable for different brands and configurations.Q: When should calibration of the tester be performed, and is it automatic?
A: The tester supports both automatic and manual calibration, ensuring ongoing accuracy. It's recommended to calibrate the device periodically as outlined in standard laboratory practices or before critical testing batches.Q: Where is the data from filter integrity tests stored, and how can it be accessed?
A: Test data is automatically stored in the device, which holds up to 100,000 records. Data can be accessed and managed through the touchscreen interface, and transferred via USB, RS232, or Ethernet ports for further analysis or archiving.Q: What processes ensure compliance with industry standards such as CFR21 Part 11 and GMP?
A: The V6.5 Filter Integrity Tester includes features like password protection, audit trail records, and controlled access settings. These functions, along with automatic data storage and secure printouts, ensure full compliance with CFR21 Part 11, GMP, and GLP guidelines.Q: How do users benefit from the built-in thermal printer and large touchscreen display?
A: The integrated thermal printer delivers instant, legible test reports, supporting immediate documentation needs. The 7-inch color touchscreen enhances usability by allowing fast parameter input and clear viewing of test results in multiple languages.Q: What makes the V6.5 suitable for use in Indian laboratories and pharmaceutical quality control environments?
A: Designed for tabletop use, the V6.5 operates on standard Indian power supplies (AC 220V 10%, 50/60 Hz), maintains low noise, and sustains performance in laboratory humidity and temperature conditions. Its integration capabilities and compliance with global standards make it an excellent choice for distributors, manufacturers, and service providers across India.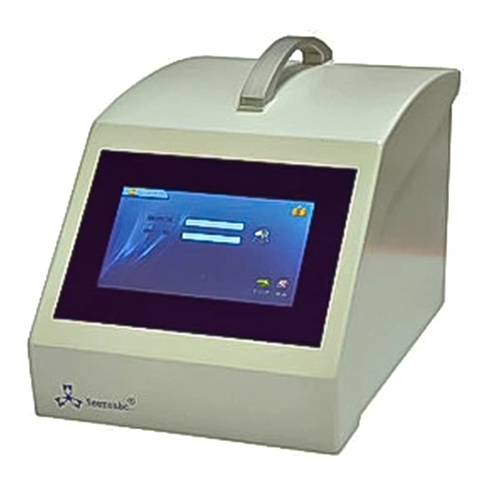

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Filter Integrity Tester Category
V8.0 Forward Flow Fest Filter Integrity
Test Range : 01000 mL/min
Usage : Laboratory, Industrial
Power Supply : 220V AC 10%, 50Hz
Frequency : 50 Hz
Max Height : 350 mm
Application : Filter membrane and cartridge integrity testing
V4.0 Full-Automatic Filter Integrity Tester Integtest
Test Range : 09999 mbar
Usage : Filter validation and routine integrity testing
Power Supply : 220V AC, 50/60Hz
Frequency : 50/60Hz
Max Height : 380 mm
Application : Filter integrity testing for pharmaceutical and laboratory filters
V1.1 Full-Automatic Filter Integrity Tester Integtest
Test Range : 06000 mbar
Usage : Filter integrity (bubble point, diffusion, water intrusion tests)
Power Supply : AC 220V 10%, 50/60Hz
Frequency : 50/60 Hz
Max Height : 35 cm
Application : Integrity testing of membrane filters in pharmaceuticals, food & beverage, biological and laboratories

 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese