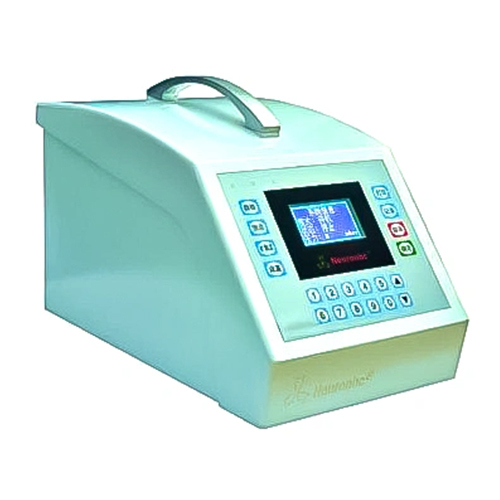
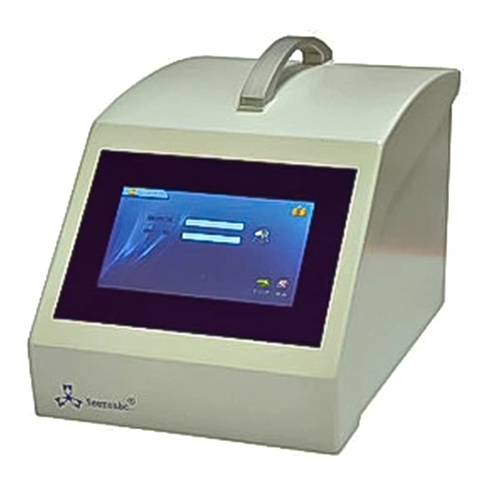
V4.0 Full-Automatic Filter Integrity Tester Integtest
V4.0 Full-Automatic Filter Integrity Tester Integtest Specification
- Response Time
- 1 sec
- Interface Type
- USB, RS232, Ethernet
- Frequency
- 50/60Hz
- Operating Voltage
- AC 220V 10%, 50/60Hz
- Number of Specimens
- 1
- Specimen Size
- Compatible with standard filter sizes
- Application
- Filter integrity testing for pharmaceutical and laboratory filters
- Accuracy
- 0.5% F.S.
- Automation Grade
- Full-Automatic
- Gas Pressure
- 0.10.7 MPa
- Display Type
- 7 TFT Color Touch Screen
- Power Supply
- 220V AC, 50/60Hz
- Max Height
- 380 mm
- Mounting Type
- Benchtop
- Temperature
- 5C40C
- Features
- Automatic data storage, real-time graph display, printer output, multi-language support
- Equipment Type
- Full-Automatic Filter Integrity Tester
- Port Size
- 8 mm
- Measuring Range
- 09999 mbar
- Humidity
- 10%85% RH (non-condensing)
- Test Range
- 09999 mbar
- Resolution
- 0.01 mbar
- Usage
- Filter validation and routine integrity testing
- Capacity
- One filter per test
- Machine Weight
- 16 kg
- Test Speed
- Fast (Full-Automatic)
- Test Width
- 120 mm
- Test Stroke
- Adjustable, up to 300 mm
- Control Mode
- Touch Screen (HMI) & PLC Control
- Data Storage Capacity
- >10000 test results
- Printer Interface
- Thermal printer built-in
- Safety Protection
- Over-pressure, over-temperature, leak detection, emergency stop
- Material of Construction
- High-grade stainless steel with ABS enclosure
- Alarm Function
- Audible and visual alarms for faults and limit exceedance
- Operating Environment
- Laboratory & cleanroom compatible
- Screen Saver
- Programmable auto screen-off
- Ambient Noise Level
- 65 dB(A)
- Software Update
- USB upgradable firmware
- Compliance Standards
- Meets GMP/GLP requirements, CE Certified
- Calibration
- Automated self-calibration function
- Testing Methods
- Bubble Point, Diffusion, Water Intrusion, and Pressure Hold
- Test Result Output
- PDF and CSV report export
- Language Support
- Multi-language option available
About V4.0 Full-Automatic Filter Integrity Tester Integtest
Main features
- Display of test data and curves in real time, monitoring the whole process of testing.
- Provides automatic printing set function, the user can operate more simple and convenient.
- Water Based test (WH) for Hydrophobic Filter: using purified water as test liquid instead of IPA and Ethanol, thus it can avoid trace quantity of ethanol or IPA contamination when testing filters.
- The instrument can store 500 history record and curve.
Applied range
- Disc Membrane:25mm-300mm;
- Standard Cartridge:2.5- 40
- Capsule & Mini Catridge
- Air filter test :2.5- 40
Advanced Testing Capabilities
The Integtest V4.0 offers comprehensive filter integrity testing with Bubble Point, Diffusion, Water Intrusion, and Pressure Hold methods. Its versatility ensures compatibility with various pharmaceutical and laboratory filter types, supporting a wide range of routine and validation requirements. Reliable automated processes and real-time graph displays provide robust data for analytical and quality control applications.
User-Friendly and Efficient Operation
Featuring a vibrant 7-inch color touch screen and PLC control, the Integtest V4.0 streamlines workflows for operators. The intuitive interface supports multiple languages, making it accessible to diverse laboratory teams. Automated data storage, rapid response times, and programmable screen saver functions further enhance daily productivity.
Compliance and Safety Assured
Engineered to meet stringent GMP/GLP regulations and CE certification, the tester incorporates advanced safety features such as audible/visual alarms, over-pressure protection, leak detection, and an emergency stop. Automated self-calibration ensures ongoing accuracy and regulatory compliance, establishing trust in critical laboratory and pharmaceutical settings.
FAQ's of V4.0 Full-Automatic Filter Integrity Tester Integtest:
Q: How does the V4.0 Full-Automatic Filter Integrity Tester perform filter integrity testing?
A: The tester applies advanced methods such as Bubble Point, Diffusion, Water Intrusion, and Pressure Hold to comprehensively validate filter integrity. Tests are conducted automatically, ensuring repeatability and highly accurate results for various standard filter sizes.Q: What is the process for exporting test results from the Integtest system?
A: Test data can be seamlessly exported in PDF or CSV formats via the built-in thermal printer or through standard connectivity options, including USB, RS232, and Ethernet interfaces, enabling secure data management and easy integration into laboratory information systems.Q: When should the calibration of the integrity tester be performed?
A: The V4.0 tester features an automated self-calibration function, which maintains accuracy and compliance. Regular system prompts and user-defined intervals ensure that calibrations are timely and effectively managed with minimal operator intervention.Q: Where is the Integtest suitable for installation and operation?
A: Engineered for laboratory and cleanroom environments, the unit is benchtop-mounted and operates efficiently within 5C-40C and 10%-85% RH (non-condensing) ranges, making it well-suited for pharmaceutical and research facilities across India.Q: How is safety maintained during the operation of the integrity tester?
A: Safety is prioritized through comprehensive protections like over-pressure, over-temperature, leak detection, an emergency stop feature, and auditory/visual alarms, all designed to prevent faults or limit exceedances during operation.Q: What are the primary benefits of using the V4.0 Full-Automatic Filter Integrity Tester?
A: Users benefit from rapid, fully-automated testing, robust data storage, real-time graph visualization, multi-language support, easy firmware upgrades, and reliable compliance with GMP/GLP and CE certification. This results in improved efficiency, traceability, and regulatory assurance for filter validation tasks.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Filter Integrity Tester Category
V8.0 Forward Flow Fest Filter Integrity
Interface Type : RS232, USB
Frequency : 50 Hz
Response Time : 2 s
Operating Voltage : 220V/50Hz
Number of Specimens : 1 sample at a time
Application : Filter membrane and cartridge integrity testing
V1.1 Full-Automatic Filter Integrity Tester Integtest
Interface Type : USB, Ethernet
Frequency : 50/60 Hz
Response Time : <2 seconds
Operating Voltage : 220V AC
Number of Specimens : Single or multiple
Application : Integrity testing of membrane filters in pharmaceuticals, food & beverage, biological and laboratories
V6.5 Filter Integrity Tester Integtest
Interface Type : USB, RS232, Ethernet
Frequency : 50/60 Hz
Response Time : 1 second
Operating Voltage : AC 220V 10%, 50/60 Hz
Number of Specimens : Single filter or filter assembly
Application : Integrity testing of hydrophilic and hydrophobic membrane filters

 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese